What are the laws of conservation for particles?
The idea is that any particle interaction must not change the total energy, total mass, and total charge of particles. The property that describes this exchange of energy and mass is called the laws of conservation, also known as ‘the particle physics conservation laws’ or ‘the conservation laws in nuclear physics’. They are:
- Mass-energy conservation: the mass and energy of particles before and after the exchange must be the same.
- Momentum conservation: the velocity multiplied by the mass of particles before and after the exchange must be the same. If the product of the mass per velocity is the momentum, then we can rephrase this as the total momentum of the system’s particles before and after the impact are the same.
- Baryon number conservation: the total baryon number of the particles before and after the exchange is the same.
- Lepton number conservation: the total lepton number of the particles before and after the exchange is the same.
- Charge conservation: the total charge of the particles before and after the exchange is the same.
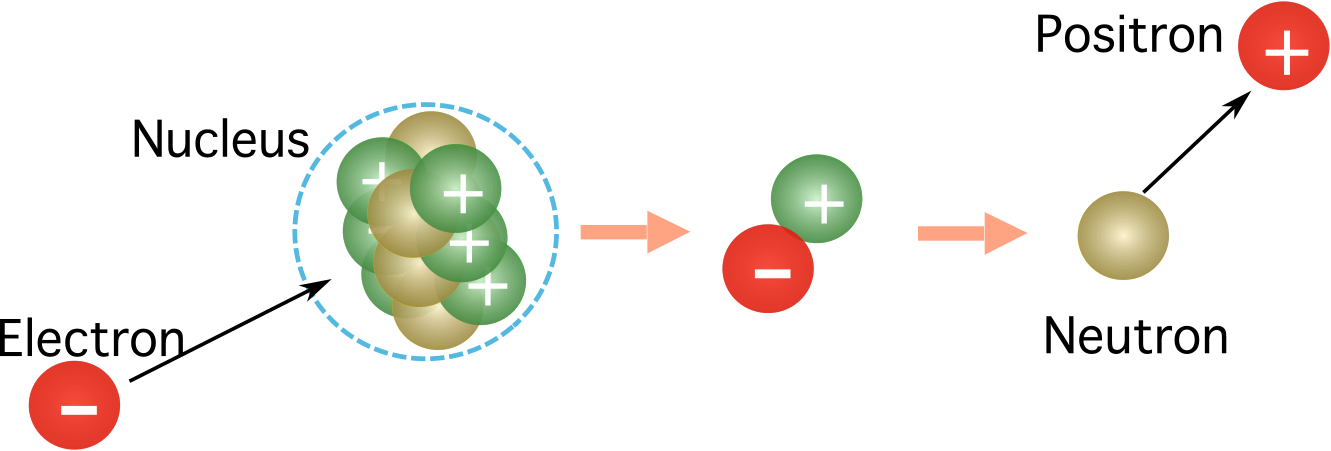
Figure 1. The capture of an electron by the nucleus. While the electron charge nullifies the proton charge, the positive charge is released as a positron. The mass is conserved, as the neutron has an equal mass to the proton, and the positron has the same mass as the electron. Source: Manuel R. Camacho, StudySmarter.
Conservation in proton to neutron nuclear reactions
Let’s consider an example of elemental charge and baryon number and lepton conservation in a weak interaction process (where the weak nuclear force intervenes).
In nuclear physics, for a proton to convert into a neutron, it must lose its charge while maintaining its baryon number. The proton to neutron process is:
\[^1_1{p} \rightarrow ^1_0{n}\]
The proton must lose its positive charge. However, the conservation law states that the total charge before and after the conversion must be the same.
\[\text{Total charge beginning = Total charge ending}\]
In this case, the total charge of the proton is 1, while the total neutron charge is 0.
\[^{\qquad \quad 1}_{charge = 1}{p} \rightarrow ^{\qquad \quad 1}_{charge = 0}{n}\]
Protons are composed of two up particles and one down particle, with charges of +⅔ and -⅓. The neutron, by contrast, consists of two down particles and one up particle.
\[p = (udu) = \Big(\frac{2}{3} - \frac{1}{3} + \frac{2}{3} \Big) = 1\]
\[n = (dud) = \Big( -\frac{1}{3} +\frac{2}{3} -\frac{1}{3} \Big) = 0\]
The proton must, therefore, lose 3⁄3 of the charge. The particle that needs to change is one of the proton’s up quarks, so the charges cancel each other out: u → d = ⅔ → -⅓. The sum for the proton will be ⅔-⅓-⅓ = 0.
The only way to achieve this is by releasing a charge of 1. A particle must, therefore, be released. In this case, the proton emits a positron.
\[^{\qquad \quad 1}_{charge = 1}{p} \rightarrow ^{\qquad \quad 1}_{charge = 0}{n} + ^{\qquad \quad 0}_{charge= 1}{e}^+\]
Now the charges are balanced and conserved. Before the process, we had a charge of 1, and afterwards, we still have a charge of 1.
The positron has a baryon number of zero, while the proton and the neutron have a baryon number of one, thus conserving the baryon number.
\[\text{Baryon number beginning = Baryon number ending}\]
\[_{\text{Baryon number = 1}}{p} \rightarrow ^{\text{Baryon number = 1}}{n} + _{\text{Baryon number = 0}}{e}^+\]
The electron is a lepton. Every lepton has a lepton number, as shown in the following table.
| Table 1. Leptons |
|---|
| Particle | Electrical charge | Lepton number | Antiparticle | Electrical charge | Lepton number |
| Electron | -1 | 1 | Positron | 1 | -1 |
| Electron neutrino | 0 | 1 | Anti-electron neutrino | 0 | -1 |
| Muon | -1 | 1 | Anti-muon | 1 | -1 |
| Muon neutrino | 0 | 1 | Anti-muon neutrino | 0 | -1 |
| Tau | -1 | 1 | Anti-Tau | 1 | -1 |
| Tau neutrino | 0 | 1 | Anti-Tau neutrino | 0 | -1 |
In the formula for the proton to neutron reaction, there is an abnormality. The proton and the neutron each have a lepton number of 0, but the positron has a lepton number of -1.
\[(\text{lepton number} = 0)p \rightarrow (\text{lepton number }= 0)n + (\text{lepton number} = -1)e^+\]
To balance the lepton number, we need to add another particle with a lepton number of 1 and a charge of 0. The particle to be added is an electron neutrino vε
\(p \rightarrow n + e^+ + v_{\varepsilon}\)
This can be summarised as follows:
\(^1_1p \rightarrow ^1_0n + ^0_1e^+ +v_{\varepsilon}\)
In this reaction, the baryon number is conserved, the charge is conserved, and the lepton number is also conserved.
The proton to neutron reaction can also be summarised as the transformation of one of the proton’s up quarks into a down quark, a positron, and a neutrino.
\(u \rightarrow d + e^+ + v_{\varepsilon}\)
Here is the reaction as represented by a Feynman diagram:
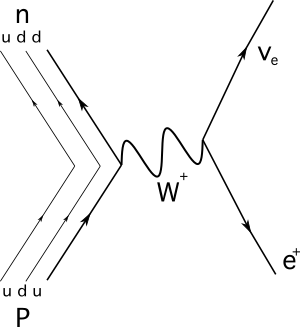
Figure 2. Proton to neutron decay. Source: Manuel R. Camacho, StudySmarter.
The particle that acts as a mediator in this reaction is a plus W boson.
Conservation in energy and momentum exchanges
Particles conserve both their momentum and their energy in quantum mechanics, as was observed in early experiments using x-rays. American physicist Arthur Compton noticed these effects as a decrease in wavelength and a scattering of photons. He theorised that the decrease in wavelength and the scattering were caused by the material’s electrons. The collisions cause the photons to scatter at an angle and reduce their energy by giving it to the electrons, an effect that is known as the Compton effect. Set the following example.
A photon with a wavelength of 0.03nm impacts an electron and changes its wavelength to 1nm. How much energy was given to the electron if the electron had an initial energy of Ei?
The energy of a photon is calculated using the energy-photon equation as below:
\(E = h \cdot f\)
Here, f is the frequency measured in Hertz, while h is the Planck constant with a value of 6.63⋅10-34 m2⋅kg/s. We need to find the frequency of a given wavelength, using the formula below, where c is the speed of light in metres per second.
\(f = \frac{c}{\lambda}\)
The energy given to the electron will be equal to the difference of the energies before and after the collision or ΔE = Ei - Ef. The wavelength contraction was equal to 0.07nm, as you can see below.
\(\Delta E = E_i - E_f = \frac{h \cdot c}{\lambda_i - \lambda_f}\)
\(E = h \cdot \frac{c}{\Delta \lambda} = \frac{(6.63 \cdot 10^{-34} m^2 \space kg/s) \cdot (3 \cdot 10^8 m/s)}{(0.07 \cdot 10^{-9} m)} = 2.8 \cdot 10^{-15} Joules\)
The energy transferred to the electron with an initial energy of Ei is:
\(E_{Electron} = E_i+ E = E_i + (2.8 \cdot 10^{-15}) Joules\)
The Compton effect is important as it provides information about the momentum of a photon, which is given as the Planck constant divided by the photon’s wavelength:
The Compton effect was important as it gave information about the momentum of a photon, where the momentum was given as the Planck constant divided by the photon’s wavelength as below.
\(\text{Photon momentum} = \frac{h}{\lambda}\)
Conservation Laws - Key takeaways
- Particles obey the laws of conservation.
- The conservation laws state that energy, mass, momentum, and numbers of the particles must all be conserved.
- In order for the conservation to be possible, the particles must release energy or other particles that balance their numbers and properties.









