What is the oil drop experiment?
The oil-drop experiment was conducted by Robert Millikan and Harvey Fletcher in 1909 in Ryerson Physical Laboratory at the University of Chicago. The purpose of the experiment was to measure the charge of a single electron.
Some earlier experiments, for instance, by J. J. Thomson, had the same goal, but his approach could only measure the charge of an electron to the predicted order of magnitude. The American physicist Robert Millikan eventually succeeded with the help of the oil-drop experiment.
The process of the oil-drop experiment
The experiment featured two horizontal metal plates on top of each other and some insulating material in between them. Four holes were cut into the insulating material, three of them for introducing light and one to allow viewing through a microscope.
A potential difference was applied across the two plates to form a uniform electric field between the plates. As oil drops were sprayed, some of them were electrically charged due to friction with the nozzle. An ionising radiation source, such as an x-ray tube, might also be used as an alternative to charging the drops.
Atomised oil drops were sprayed into a chamber above the plates. Oil is used instead of water because it doesn’t evaporate quickly, which allows the mass to remain approximately constant.
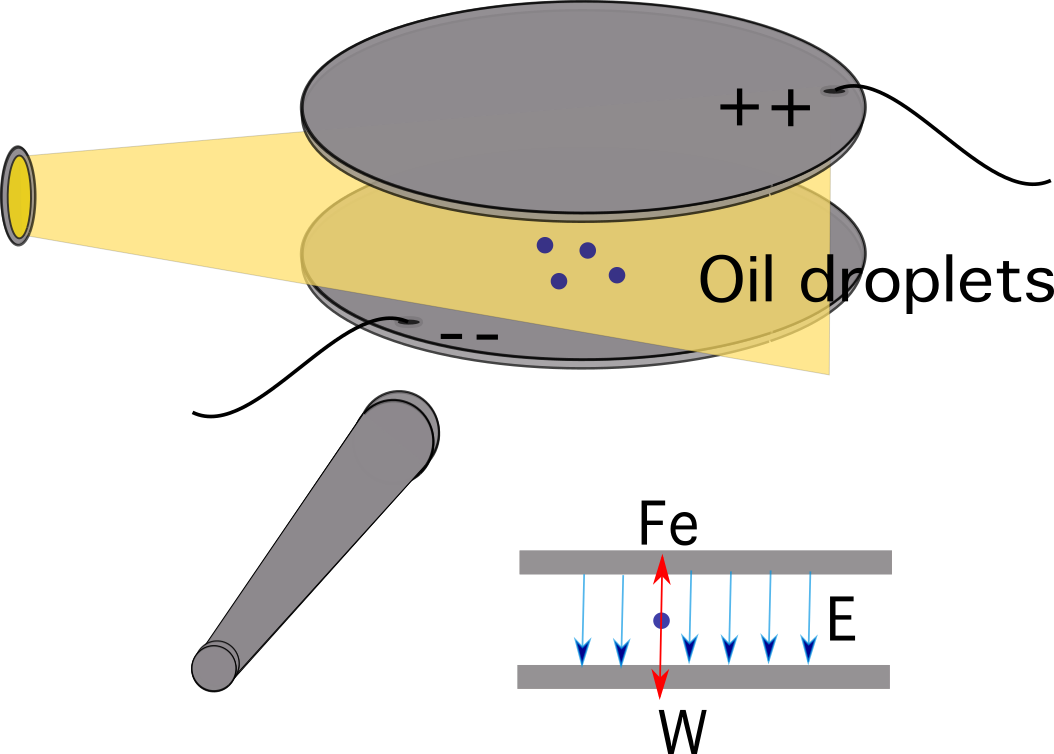
Figure 2. A simplified diagram of Millikan’s oil-drop experiment. Source: Manuel R. Camacho, StudySmarter.For the process, the following steps were followed:
- While the electric field was turned off, the oil drops were introduced between the plates and, because of the friction with the air in the chamber, they attained terminal velocity pretty quickly.
- The electric field was turned on, and the oil drops that were charged began to rise. This happened when the electrical field was strong enough and caused the upwards electrical force FE to be greater than the gravitational force Fg.
- One suitable drop was selected to continue the experiment while keeping this selected drop in the middle by alternately switching off the voltage until the other drops came down.
- The drop is allowed to fall at its terminal velocity. This means that the net force on the drop is zero, meaning the force of gravity on the drop and the force of the electric field on the drop are equal.
So, how does this help to determine the charge of a single electron? Let’s look at the equations Millikan and Fletcher used to understand this concept.
As we noted earlier, the force of gravity is equal to the force of the electric field when the drop is falling at its terminal velocity.
\[m_{drop} \cdot g = q_e \cdot E\]
In this equation:
- m is the mass of the drop in grams.
- g is the gravitational constant, 9.8 m/s2, at the earth’s surface.
- qe is the charge of the electron in Coulombs.
- E is the electric field in Newtons/Coulombs.
A microscope can detect the droplets as reflected light points, but they are too tiny to estimate their size and mass by direct measurements. When the voltage is turned off, the mass of the drop is determined by how quickly it falls. Because of air resistance, the more massive ones fall more quickly than the less massive ones, revealing their mass by advanced sedimentation calculations.
We stated that the voltage (V) is adjusted to balance the drop’s weight, and since the electric field is produced by the applied voltage, it can be calculated as shown below.
\[E = \frac{V}{d}\]
Here, d is the distance between the plates in metres.
Once the mass of the drop is known, the charge of the electron can be calculated using the rearranged equation below.
\[q = \frac{m_{drop} \cdot g}{E} = \frac{m_{drop} \cdot g \cdot d}{V}\]
Here, V is the voltage that holds the drop in place.
Millikan had measured the charge of the electron qe to a precision of 1%, and within a few years, he increased this by a factor of 10 to a value of -1.60⋅10-19 C. He also noticed that all charges were multiples of the fundamental electron charge and that electrons may be added or subtracted from the drops at any time.
How did Millikan’s experiments affect photoelectricity?
Having carried out the oil-drop experiment, Millikan released a report, hoping to invalidate Albert Einstein’s 1905 theory of the photoelectric effect. This said that the electrons are ejected from a metal surface when they are impacted by light at certain wavelengths.
Millikan believed that if the photoelectric effect experiment was done correctly, no electrons would be released. He carefully carried out his experiment in a vacuum, ensuring that nothing in the atmosphere could interfere with the set-up or facilitate the formation of a current.
However, contrary to his intentions, he ended up proving the photoelectric effect, which led to Einstein receiving the 1921 Nobel Prize for the theory of photoelectric effect and to Millikan himself receiving the 1923 Nobel Prize for his work on determining the charge of an electron and proving the theory of the photoelectric effect.
Millikan’s Experiment - Key takeaways
- The oil-drop experiment was conducted by Robert Millikan and Harvey Fletcher for the purpose of determining the charge of a single electron.
- Millikan suggested that if the photoelectric effect experiment was done correctly, no electrons would be released. However, as a result of his experiment, he ended up proving the theory of the photoelectric effect to be correct.
- Millikan determined that the charge of a single electron is -1.60⋅10-19C.
- The results of the oil-drop experiment also determined that all charges were multiples of the fundamental electron charge.
- The oil-drop experiment featured two horizontal metal plates on top of each other and some insulating material in between them. Four holes were cut into the insulating material, three of them for introducing light and one to allow viewing through a microscope.








