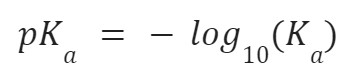Acid dissociation constant definition
We know that an acid dissociates into an H+ ion and the corresponding anion. We also know that the reaction of acid and water is an ionisation reaction. These reactions are reversible and reach an equilibrium state where the rates of forward and backward reactions are the same. The equilibrium constant calculated for an acid solution at equilibrium is called the acid dissociation constant.
Acid dissociation constant is a term used to quantitatively measure the strength of an acid in a solution.
You must remember how to find the equilibrium constant of a chemical reaction. The dissociation constant is nothing else but the equilibrium constant of the ionisation reaction of any acid in a solution. For any acid HA, the equilibrium reaction for the solution of HA would be:
Here, H+ is a Hydron, or Hydrogen ion, which gives any acid its acidic nature. The anion formed (A-) after HA releases H+. This is called the conjugate base of the acid HA. As you might remember, a solution is said to be in a state of equilibrium when the rate of the forward reaction is equal to the rate of the backward reaction. The acid dissociation constant is calculated at equilibrium. It is denoted by Ka.
Remember that square brackets refer to the concentration of the molecule/ion.
Looking at the formula for Ka, it might seem that it depends on the concentration of the acid/ions in the solution, but that is not true. The value of Ka for an acid is fixed, and does not depend on the concentration of the acid. Ka varies only with temperature.
Usually, the value of Ka is very large, so a logarithm of Ka is taken. This is called pKa.
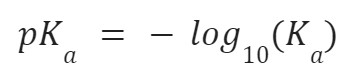
The acid dissociation constant is sometimes also called acid ionisation constant, or acidity constant.
pH of a solution
You might have come across the term pH while studying Chemistry. pH is a measure of the acidic strength of a solution. For the sake of understanding, it can be considered as a scaled quantity of concentration of H+ ions in a solution.
Find out the pH of a solution with H+ ion concentration equal to 10-7.
Solution
Given:
[H+] = 10-7
we know that:
pH = -log10[H+]
∴ pH = -log10(10-7)
pH = 7
Common exam questions might give you information about the pH of a solution and ask you to find the Ka of a particular acid. It may be the other way round; they'll give you information about Ka of an acid, and ask you to find the pH of a solution of that acid.
The pH of a solution can range from 0 to 14. Solutions with pH less than 7 are acidic, while those with a pH above 7 are basic. The characteristic sour taste of lemons is due to the presence of citric acid, giving it a pH of ≈ 2. The black coffee you have in the morning has a pH of ≈ 5. Saliva has a pH of ≈ 6.6. On the basic side, the baking soda you have in the kitchen has a pH of 9.5. Liquid drain cleaners have a pH of ≈ 14 (very strong, which is why we use them with care; they can hurt skin).
Did you know that the pH of human blood ranges between 7.35 and 7.45; i.e. it is slightly on the basic side. This is necessary to balance out the human body's various metabolic processes which produce acids.
It's easy to confuse pH and pKa when you're learning about them for the first time. For the sake of clarity, pKa is for an acid, and pH is for a solution. pKa depends on the temperature of the acid/solution, while pH depends on the concentration of H+ ions in the solution.
For the same amount of acids added to 2 solutions, the pH of the solution with the stronger acid (higher pKa) will be lower (more acidic).
Acid dissociation constant of HCl
A common example of a strong acid is hydrochloric acid (HCl). For HCl, the ionisation reaction would be written like this:
The equilibrium constant for the above reaction would be calculated as:
The H+ ions are transferred to H2O and form hydronium ions (H3O+). This is because free H+ ions do not exist in an aqueous solution. For simplicity, we can replace [H3O+] with [H+]. Also, [H2O] is equal to 1 as the concentration of water is 1. Hence, the equation for Ka becomes:
The addition of H+ ion to a water molecule is called protonation of water. Can you guess why? (Hint: it's in the name, 'protonation').
HCl is a strong acid. This means that it dissociates completely into H+ and Cl- ions in solution. For example, if 1 mol of HCl is added to 1 L of water, it will give out 1 mol of H+ ions and 1 mol of Cl- ions.
This can be represented with the help of an ICE table (Initial, Change, Equilibrium), where we can write the concentrations of the reactants and the products of the reaction at the initial stage (Initial), the change in their concentration from initial stage till equilibrium (Change), and their final concentrations at equilibrium (Equilibrium).
| [HCl] | [H+] | [Cl-] |
| Initial | 1 | 0 | 0 |
| Change | -1 | 1 | 1 |
| Equilibrium | negligible | 1 | 1 |
In the ICE table for the ionisation reaction of HCl, we have written the concentrations of the reactants and the products at the initial stage, and the final stage. We can now substitute these values in the formula for Ka for HCl.
There is a very small number in the denominator, as there is always a very small amount of undissociated acid at equilibrium. Therefore, the value of Ka for strong acids is very large. Ka for HCl is very high, at 1.3 x 106.
Examples of other strong acids include:
- Perchloric acid (Superacid) - HClO4 (Ka > 1.0 x 109)
- Nitric acid - HNO3 (Ka = 2.4 x 101)
- Hydrobromic acid - HBr (Ka = 1.0 x 109)
- Hydroiodic acid - HI (Ka = 3.2 x 109)
- Sulfuric acid - H2SO4 (Ka = 1.0 x 103)
In this subsection, we learnt that strong acids are so-called because they dissociate completely into H+ ions and the corresponding conjugate bases.
But what about acids that cannot dissociate completely in a solution? Those acids are called weak acids.
Determination of the dissociation constant of a weak acid
Let us consider an acid HB. The ionisation reaction equation will be written as:
Consider HB as a weak acid. Weak acids do not dissociate completely, i.e. if 1 mol of HB is added to water, at equilibrium the solution will have less than 1 mol of H+ and B-, and will also contain a certain concentration of undissociated HB at equilibrium (unlike the case of strong acid, which only contained H+ and the conjugate base at equilibrium). Due to incomplete dissociation/ionisation of the weak acid, the solution is not as acidic as it would be if an equal quantity of a strong acid was added to the solution.
Let us take the example of acetic acid (CH3COOH), a weak acid. Consider adding 1 mol of acetic acid to 1 L of water. The concentration of acetic acid would be 1 mol/L or 1M. The equation of the reaction can be written as:
At equilibrium, the Ka for this equation can be written as:
Acetic acid is an organic acid that is the primary acid in vinegar. Besides that, it is also found in apples, strawberries, and grapes.
Just as we drew an ICE table for the ionisation of HCl, we can draw the ICE table for the ionisation of acetic acid. This will help us better understand the concentrations of the reactants and the products at different stages of the reaction.
| [CH3COOH] | [H+] | [CH3COOH-] |
| Initial | 1 | 0 | 0 |
| Change | -x | +x | +x |
| Equilibrium | 1-x | x | x |
Weak acids do not dissociate completely, which is why the change in concentration of CH3COOH is -x. We use the variable x because we do not know how much the acid will dissociate, and hence how many H+ ions or CH3COO- ions there will be in the solution.
You know that acetic acid is a weak acid. You have also understood that weak acids do not ionise completely in a solution. Therefore, in the equation of Ka for acetic acid, the concentration of the H+ ions and the conjugate base is less than 1M, while the concentration of the reactants is a significant value, as we know there is still undissociated acid at equilibrium (unlike the case of strong acids). So, for weak acids, the numerator while calculating Ka is small, whilst the denominator is big. This implies that the value of Ka for weak acids is small. Ka for acetic acid is 1.8 × 10−5 .
Examples of other weak acids are:
- Methanoic acid - HCOOH (Ka = 1.78 × 10−4)
- Benzoic acid - C6H5COOH (Ka = 6.3 × 10−5)
- Hypochlorous acid - HClO (Ka = 2.9 × 10−8)
- Hydrocyanic acid - HCN (Ka = 6.2 × 10−10)
1 mol of acetic acid is added to 1 L of water. Calculate the pH of the solution at equilibrium.
Solution
Given:
CH3COOH = 1 mol
Water = 1L
∴ [CH3COOH ] = = 1M
We know that,
To calculate pH, we need to know the concentration of H+ ions in the solution at equilibrium. To do this, we shall draw an ICE table for the reaction.
| [CH3COOH] | [H+] | [CH3COO-] |
| Initial | 1 | 0 | 0 |
| Change | -x | +x | +x |
| Equilibrium | 1-x | x | x |
Since acetic acid is a weak acid and so it wouldn't have dissociated very much, we can assume that its equilibrium concentration is approximately that of the original concentration.
Note that we can replace [CH3COO-] with [H+] since both are equal.
Now that we know the concentration of H+ ions in solution, we can calculate the pH of the solution by taking its log.
Acid Dissociation Constant - Key takeaways
- Acid dissociation constant (Ka) is used to quantitatively measure the strength of an acid.
- Acid dissociation constant is nothing but the equilibrium constant calculated for the ionisation of an acid.
- An acid HA dissociates into the hydrogen ion (H+) and the conjugate base of that acid (A-).
- The value of Ka is usually very large or small. That is why it is scaled down by taking a negative log; pKa = -log10Ka.
- pH determines how acidic a solution is. pH = -log10[H+].
- pH ranges from 0-14. 7 is neutral. Below 7 is acidic, above 7 is basic.
- Strong acids are those acids that dissociate completely. Weak acids are those that do not dissociate completely.
- Strong acids have a high Ka value. Weak acids have a small Ka value.
- An acid dissociation constant table (or ICE table) can help understand the concentrations of the products and reactants at the initial stage of the reaction, and at equilibrium.