Now imagine that the aisle is an atom, the child is a nucleus and the chocolates are the electrons. What is the role of the mom? Let us save that bit for later.
The nucleus attracts the electrons of an atom (and vice versa). When it succeeds in attracting a new electron, the atom becomes a negatively charged ion: an anion. You already know the change in the charge on a neutral atom when an atom is accepted or given. But have you ever wondered... How is the energy of the atom affected? What factors govern the change in energy?
All these questions on what happens to the change in energy once an electron is added to an atom are dealt with by the concept of "electron affinity".
- In this article we will go over the definition of electron affinity, and how it differs from electronegativity.
- What the first electron affinity is and what the successive electron affinities are.
- The factors that influence electron affinity, as well as the periodic trends observed.
- along with Group 16 and 17 trends
- Comparison of electron affinities of F and Cl of group 17
- Examples of electron affinities in different contexts.
Electron Affinity Definition
So, what is electron affinity? Electron affinity is a measure of the attraction between the nucleus and the incoming electron. If you need a down-to-earth example, remember the boy and the chocolates. With this example, we understood the word affinity means attraction. The attraction we are discussing, in this case, is between the nucleus and the incoming electron.
The electron affinity of an atom is defined as the change in the energy when an electron is added to a neutral gaseous atom forming a negatively charged ion (anion).
The electron affinity values can be exothermic or endothermic, depending upon when you are adding the electrons and what you are adding the electrons to. For example:
- Are you adding an electron to the neutral atom, or to a negative ion (anion)?
- Are you adding an electron to a metal or a non-metal?
- Is the atom happy to accept the electron or are you forcing an electron into it while it is reluctant?
Based on the answers to these questions, we can predict if the addition of the electron is associated with the release or the absorption of energy.
Did you know you can also calculate the electron affinities for molecules?
Electron affinity is a physical chemistry term that doesn't always concern just single atoms, but often whole molecules. For example, the organic molecule benzene has an electron affinity of -68 kJ mol-1. When we define electron affinity for a molecule, it is the amount of energy released to convert it into a molecular ion.
The generation of molecular ions is of great importance in the identification of organic and inorganic compounds and also their structural elucidation (determining how the atoms are bonded with each other).
The tool employed to generate negatively charged molecular ions is called negative ion mode mass spectrometry. There are several techniques for the ionization of molecules into corresponding ions, but one of the most popular techniques these days is the Electron Spray Ionisation technique (ESI).
Using the ESI, ions are generated. Once the ions are formed, the spectrometer analyses the mass-to-charge ratio (m/z) and presents it to us in the form of a graph called a mass spectrum. In a mass spectrum, the m/z ratio is plotted against the relative intensity. The height of each peak represents the relative abundance.
Given below is an example of a mass spectrum of acetone. The most abundant ion gives the base peak (the longest red vertical line).
 Fig. 1: Example of a mass spectrum of acetone.
Fig. 1: Example of a mass spectrum of acetone.
Electron Affinity Equation
The first electron affinity (EA1) is the energy change associated with the addition of a mole of electrons to a mole of a neutral atom (in the gaseous state) to form one mole of gaseous anions under standard conditions. It is measured in kJ/mol.
In other words, you take a neutral atom, add an electron and energy is released as a result of this addition. This release of energy is the first electron affinity. First electron affinities are exothermic and negative in general.
Let us put the definition of first electron affinity in the form of an equation. Consider X as an atom in gaseous state. It accepts an electron and transforms into an anion.
\[ X_{(g)} + e^- = X^-_{(g)} + energy \]
Using the formula above, you can write the first electron affinity formula for any element you desire.
What if you want to add another electron to the anion [\(X^-\)], i.e. what characteristics does the second electron affinity have?
The anion already has enough electron density. It repels the next incoming electron; therefore you would need to invest energy to force an electron into the anion. This means energy is absorbed, and the process is endothermic because the anion is reluctant to take up that extra electron. Hence, the second electron affinity and the successive electron affinities are endothermic.
The equation to represent the second electron affinity of the anion \(X^-\) can be given as follows:
\[ X^-_{(g)} + e^- = X^{2-}_{(g)} \]
It is important to remember that successive electron affinities don't measure the overall energy released by the cumulative addition of all electrons, but rather just the electron at hand. For example, the second electron affinity is not the energy value of adding two electrons to an atom, but rather just the energy associated with adding a second extra electron to an ion which has already taken the first extra electron.
Factors Affecting Electron Affinity
There are four factors that influence electron affinity:
- Nuclear Charge
- Electronic Configuration
- Atomic Size
- Shielding effect
In the following diagram, you can see a summary of what conditions give a greater electron affinity. We will explain what each factor means and how it influences electron affinity in more detail below.
Nuclear Charge
Nuclear charge accounts for the positive forces in the atom, as dictated by the number of protons in the nucleus. This can be determined by the atomic number of an element.
To be more precise, we take into account the effective nuclear charge the electron would be subjected to, but for now, let us treat it as the atomic number.
The greater the nuclear charge, the stronger the pull, and the easier it is to add the incoming electron.
Electronic Configuration
Electronic configuration refers to the way electrons are arranged inside an atom, more specifically regarding the energy levels and subshells. The electronic configuration is like the postcode of an electron in an atom. It tells us which subshell of an orbit the electron occupies.
Electron configuration matters to electron affinity, as an element can only accept an electron if there is "space" for it. This means that atoms that have unfilled shells are more likely to accept extra electrons. If an element has a stable electronic arrangement such as an octet, it is highly unlikely for such a stable atom to accept an extra electron.
Atomic Size
The larger the atomic size, the greater the distance between the nucleus and the valence shell (outermost). The capacity of the nucleus to pull the electrons towards itself will be less. Thus, it is difficult to introduce an electron into the atoms with greater size, meaning- the energy released will be less.
Thus, atoms of smaller size have greater electron affinities because of the ease of placing an electron owing to the greater nuclear pull. The energy released will be high resulting in bigger electron affinity values.
Shielding effect
Here comes the role of the mom we discussed at the beginning of the article. The boy had chocolate, he wants to have more. She stops him from grabbing more by blocking his way to the chocolate shelf by standing in between.
The mom represents the set of electrons in the inner shells that stand between the nucleus and the incoming electrons. The inner electrons reduce the nuclear pull/attraction by shielding it and repelling the incoming electrons. The incoming electrons have to overcome the repulsive forces (resistance) to grab a seat in the last shell of the atom.
In short, the shielding effect decreases the attraction between the nucleus and the electron that is trying to join the atom. This is because the inner electrons have a negative charge, the same as the incoming electron, causing them to repel each other.
The greater the shielding effect, the greater the difficulty of the addition of an extra electron, and the lesser the release of energy, thus the lesser the electron affinity.
Atoms of larger size have more inner electrons, and thus greater shielding effect and lesser electron affinity values.
Trends in Electron Affinity
As with many other atomic characteristics, electron affinity also has trends of increase/decrease in the periodic table. Here we will go over what trends in electron affinity are and how the above factors affect the trends when you move around the periodic table.
Overall, the general trends are:
Going across periods from left to right, the electron affinity increases.
Going down a group, the electron affinity decreases.
Can you see how the factors which influence electron affinity, which we talked about in the section above, create the trends we observe on the periodic table?
Electron Affinity Chart
Here you can find a simple chart which visualises the trends observed with electron affinity. It is a periodic table which shows which way the electron affinities of elements decrease and increase. Can you speculate how the factors which influence electron affinity come together to create this dynamic landscape?
Electron affinity increases upwards in a group and from left to right in a period because the top elements have a smaller diameter, i.e. fewer inner electrons (less shielding effect) and the new electron would come in closer to the nucleus (higher nuclear attraction force).
Trends in Electron Affinity of Groups 16 and 17
Group-16, the oxygen family/chalcogens, and group-17, the Fluorine family/halogens, are non-metals. The oxygen family has 2 electrons short of attaining the noble gas configuration while the halogens are one electron short.
Both the groups have a good affinity towards the electrons as they are eager to achieve a full octet to attain stability. Therefore, we will look into the trends of electron affinity in Groups 16 and 17.
Let us start by comparing Oxygen from group 16 and Fluorine from group 17.
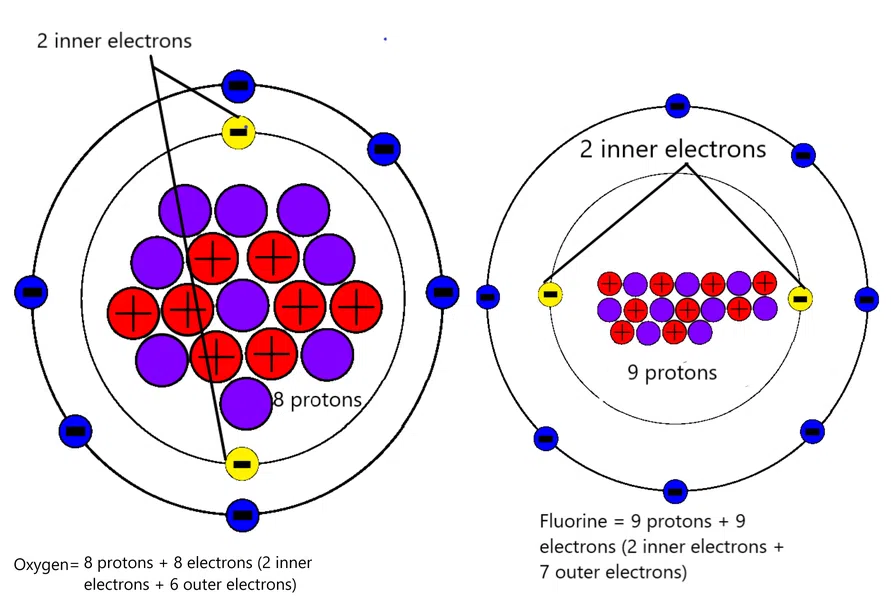
The figure demonstrates the number of protons and electrons in oxygen and Fluorine respectively. Observe that both Oxygen and Fluorine have the same number of inner electrons (coloured in yellow) but a different number of protons-oxygen has 8 while Fluorine has 9 protons.
The shielding effect is the same in both because of the same number of inner shell electrons, but since Fluorine has an extra proton as compared to that of oxygen, the nuclear charge in Fluorine is more; therefore it is easy to insert an electron in the outer shell of Fluorine.
Based on these observations, we can say that the energy released while attaching an electron is Fluorine is more than that of oxygen. Thus, Fluorine has a high electron affinity value as compared to that oxygen.
The first electron affinity of Oxygen = -142 kJ mol-1
Fluorine = - 328 kJmol-1
You should be careful while understanding the significance of the negative sign here. It is just used to represent the energy release. The magnitude of the number is considered while comparing the values ( 328 > 142 implies Eea Fluorine > Eea Oxygen)
This very same observation dictates that the electron affinity increases from left to right across a period. One more conclusion we can draw from this is that the metals (groups 1 and 2, especially) have lower electron affinity values because they are eager to get rid of electrons to achieve the noble gas configuration. So, it is difficult to squeeze an extra electron into them. You will need to invest energy to do so. Hence, the EA values will be endothermic and positive.
Given, below are the EA values of Alkali metals of Group-1.[1]
- Lithium (Li): 59.63 KJ mol-1
- Sodium (Na): 52.86 KJ mol-1
- Potassium (K): 48.38 KJ mol-1
- Rubidium (Rb): 46.88 KJ mol-1
- Caesium (Cs): 45.50 KJ mol-1
What happens to the electron affinity trend as we go down the group?
For convenience, let us again consider the group-17/halogens because they are happy to accept an electron. Generally, the electron affinity should decrease as we go down the group. But notice the EA values[2] of halogens below.
- Fluorine (F) -328 kJ·mol-1.
- Chlorine (Cl) -349 kJ·mol-1.
- Bromine (Br) -324 kJ·mol-1.
- Iodine (I) -295 kJ·mol-1.
Did you notice that naughty Fluorine is not following the usual trend? Why? Can you have a look at the factors we discussed in the previous section and deduce a reason?
Well, yes, if you are thinking that the atomic size is the reason, you are right!
The size of the Fluorine atom is less than that of Chlorine (remember? the atomic size increases down the group due to the addition of an extra shell) and the electrons are tightly packed in Fluorine. So much electron density is packed up in a small space. This causes repulsions between the existing electrons and the incoming new electron. It is a large family packed in a small house and if a new member comes, it becomes too crowded. So, they try their best to avoid it (bear in mind that this is as you compare with chlorine, in general Fluorine is happy to accept electrons).
On the other hand, chlorine has bigger room than Fluorine. The incoming electron will not experience as many repulsions as it does with Fluorine.
Now, can you tell which element has the highest electron affinity in the periodic table?
Yes, Chlorine.
Further Electron Affinity Examples
Let us explore examples of some elements along with their electron affinity values to appreciate the differences in their behaviour.
Chlorine- Halogen- Group 17
\[ Cl_{(g)} + e^- = Cl^-_{(g)} \qquad \Delta H^\circ = -349 \space kJmol^-1 \]
Sodium- Alkali metal- Group 1
\[ Na_{(g)} + e^- = Na^-_{(g)} \qquad \Delta H^\circ = 52.8 \space kJmol^-1 \]
Beryllium- Alkaline earth metal- Group 2
\[ Be_{(g)} + e^- = Be^-_{(g)} \qquad \Delta H^\circ = 0 \space kJmol^-1 \]
Neon- Noble gas- Group 0
\[ Ne_{(g)} + e^- = \qquad \Delta H^\circ = 0 \space kJmol^-1 \]
Observe that we took different elements from different groups. This is to compare and explain how different the electron affinity values are from each other across different groups. Also, the nature and physical characteristics of each element are different which can also form the basis for explaining why the electron affinity of a certain element is low or high.
Let us revisit the questions we asked in the Electron affinity definition part of this article.
1. Are you adding an electron to the neutral atom or to a negative ion (anion)?
- In all the examples, we considered neutral atoms. So, this is the first electron affinity we are referring to.
2. Are you adding an electron to a metal or a non-metal?
- Chlorine is a non-metal, eager to accept electrons. Therefore, the electron affinity (EA) value is high and negative which accounts for the release of energy.
- On the other hand, Sodium and Be are metals. While it is quite a struggle to insert an electron in sodium, it is nearly impossible to do so with Be (zero EA). The anions formed will be extremely unstable. Therefore, the values represent that EA is endothermic for both of these metals.
- Coming to Neon is a noble gas. Well, it is completely satisfied with a complete valence shell of electrons, and hence no vacant seat. Therefore, for all the noble gases, the EA values are zero.
3. Is the atom happy to accept the electron or are you forcing an electron into it while it is reluctant?
- Chlorine is happy to accept the electron, but we have to forcibly add an electron to all the others. Hence, Chlorine is the element with the highest EA not just among the others in the list, but in the entire periodic table.
Electron Affinity vs. Electronegativity
In physical chemistry, these two terms electronegativity and electron affinity come up a lot and sometimes are used in similar contexts. So, what do they mean, and what is their difference?
We know from previous sections in this article, electron affinity refers to the quantified energy released upon the addition of an electron to a gaseous atom.
On the other hand, electronegativity refers to the tendency of elements to attract and hold on to electrons. The main difference is that electronegativity refers to how the elements act how in bonded molecules, and how much of the bonded shared electrons can they hold on to. Electron affinity refers to the ability of elements to release energy, thus showing the ease and spontaneity, of adding an extra electron to the sole atom.
Another difference is that electronegativity is a value from 0 to 4.0, while electron affinity is a defined thermodynamic constant for each element in kJ per mole.
In this article, you should have understood the general concept of electron affinity and its trends. This topic might come up everywhere you look actually, and energetically these concepts will be useful to have a good grasp over.
It is interesting to note that the element with the highest electro-negativity is Fluorine and not chlorine. Fluorine has a greater tendency to accept electrons, but the ease of inserting an electron is greater in chlorine. Fluorine is like that 3-year-old who is eager to accept chocolates, but his mom doesn't allow him to. But, chlorine is like a 15-year-old. No one is stopping him from having chocolates, he has the ease and flexibility, but he doesn't want to (as compared to Fluorine).
To be precise, Electronegativity is defined as the tendency of an atom to attract a bonding pair of electrons towards itself. The difference in electronegativities of atoms connected with a bond dictates whether the bond is an ionic, polar covalent or non-polar covalent bond.
There are two scales to measure Electronegativity which are based on the concept of Electron affinity.
1. Mulliken scale
2. Pauling scale
Mulliken's scale
Mulliken's scale was developed by Robert Mulliken who observed that elements with large ionization energies have very exothermic (negative) electron affinities and have a tendency to accept electrons during chemical reactions. He proposed that electronegativity is an average of ionization energy and electron affinity.
One must note that Mulliken's scale considers the magnitude of the values, ignoring the signs(+ and -)
It is given by the formula
\(X\) = \(\frac{IE_1 + EA} {2}\)
He termed this as absolute electronegativity.
Mulliken's scale
Linus Pauling described electronegativity as the property of an atom to attract a bond pair of electrons towards itself.
Pauling's scale relates to Mulliken's scale by the expression:
\(X\) = \(\frac{IE_1 + EA} {2\times 2.8}\)
Although there are several other scales to discuss electronegativity, the Mulliken and Pauling scales are the most common ones.
Electron Affinity - Key Takeaways
- Electron affinity is the energy released (in kJ per mole) when one mole of gaseous atoms acquires a mole of electrons.
- The first electron affinity of elements is the energy released when a neutral atom gains one electron to become an anion with charge -1.
- The formula for the first electron affinity is: \( X_{(g)} + e^- = X^-_{(g)} + energy \) . The first electron affinity is exothermic and negative.
- Successive electron affinities describe the addition of a second, third... and so on, electron to the atom in question. Successive electron affinities are usually positive and endothermic.
- The factors that influence electron affinity are electronic configuration, atomic size,nuclear charge and shielding effect.
- The periodic trends of electron affinity are: going across periods from left to right, the electron affinity increases, while going down a group, the electron affinity decreases.
References
- https://www.rsc.org/periodic-table/element/3/lithium
- Harjeet Bassi, Nilpa Shah, Shelley Chu, Jim Clark, & Jim Clark , licensed under CC-BY 4.0(https://creativecommons.org/licenses/by/4.0/), Chemistry Libretexts-Electron affinity
Similar topics in Chemistry
Related topics to Physical Chemistry









