- In this article, we will talk about fullerenes.
- First of all, we will analyse the buckminsterfullerene.
- Then, to finish, we will talk about carbon nanotubes, its properties and uses.
Allotropes are different structural forms the same element can exist in.
Fullerenes, Graphite, and Diamond are allotropes of Carbon because they all are made of Carbon atoms. The difference is in the molecular structure i.e. the arrangement of atoms that make the basic repetitive structure of the entire molecule.
Large Fullerene molecules are generally composed of 6-Carbon rings, but sometimes also have 5-, or 7-Carbon, rings. The Carbon atoms in Fullerenes may be joined by single covalent bonds or double covalent bonds. Fullerenes are made synthetically and not found naturally. There are many Fullerene molecules that are possible to synthesize. The smallest fullerene molecule is made up of 20 Carbon atoms, while the largest can have 720 Carbon atoms! Let us look at some common Fullerene molecules.
Fullerenes may be found in interstellar dust, or meteors, but they're not found in the Earth's atmosphere.
The structure of fullerene
Buckminsterfullerene C60 is a Fullerene molecule made up of 60-Carbon atoms. The atoms are arranged in hexagonal and pentagonal rings. There are 20 hexagonal rings and 12 pentagonal rings, which together form a spherical structure. Look at the figure to visualize the molecule shape of Buckminsterfullerene.
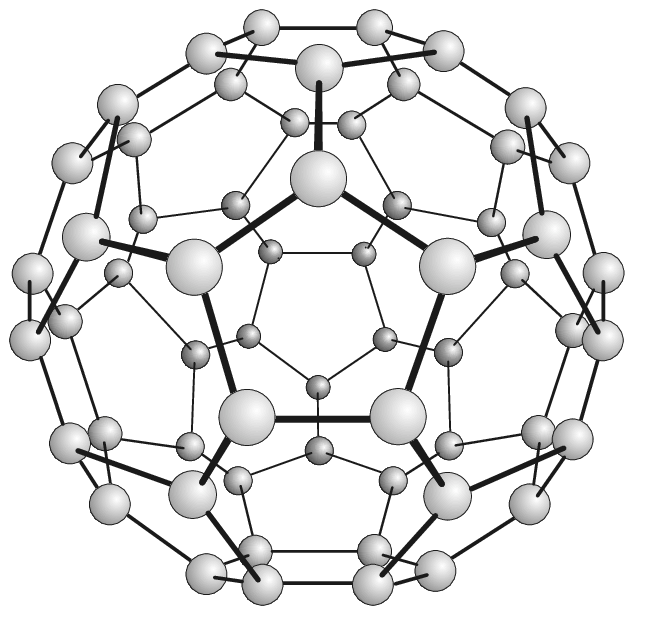
Fig. 1: Buckminsterfullerene (C60) |
ResearchGate In the figure, you should be able to see 6 hexagonal rings surrounding a pentagonal ring? Buckminsterfullerene is also called Buckyball (easier to remember, isn't it?). C60 Buckminsterfullerene is the smallest fullerene molecule in which no 2 pentagonal rings are touching each other.
The structure of Buckminsterfullerene C60 is called truncated icosahedron. This shape of the structure resembles a football, and is made of 20 regular hexagons and 12 regular pentagons. Each vertex of each polygon has a Carbon atom; and each edge of each polygon is a bond between the Carbon atoms.
Buckminsterfullerene was the first fullerene to be discovered in 1985 by Robert Curl, Harold Kroto, and Richard Smalley. They were awarded the Nobel prize in Chemistry in 1996 for the discovery of fullerenes.
In fullerene molecules, each Carbon atom makes 3 covalent bonds. The Carbon atoms share single and double covalent bonds. Double bonds are called pi-bonds (π), and the electrons shared in a π bond are called π electrons. These π electrons form an electron cloud around the entire structure of the Buckyball molecule. The molecule essentially occupies space which can accommodate this π electron cloud.
The diameter of the C60 molecule including this π electron cloud is 1.0 nm. This is also called the Van Der Waals diameter of fullerene.
 Fig. 2: Diameter of Buckminsterfullerene | ScienceDirect
Fig. 2: Diameter of Buckminsterfullerene | ScienceDirect
The diameter of the C60 molecule measured from nucleus to nucleus (excluding the π electron region) is 0.7 nm.
There are many possible fullerene molecules, but Buckminsterfullerene (C60) is most common. Another common fullerene is the C70 fullerene, consisting of 70 Carbon atoms.
Fullerenes come in many more sizes. The smallest fullerene has only 20 Carbon atoms. It is made up of pentagonal rings of Carbon atoms. Some fullerenes are made up of as many as 720 Carbon atoms - the C720 fullerene.
Properties of fullerene
Their unique molecular shape and the presence of π electron region gives them these properties:
- Buckminsterfullerene can participate in various types of chemical reactions. This comes due to its ability to both donate and accept electrons.
- It can chemically react with other species while keeping its spherical structure intact.
- It can resist high temperatures and pressures without changing chemically.
- Insoluble in water.
- Sublimation temperature of 600oC. This means that at 600oC, it changes from its solid state to its gas state directly, without changing into liquid state.
Two important properties of fullerene are its melting point ant its diameter.
Fullerene melting point
The melting point of fullerene is 280ºC.
Fullerene diameter
The fullerene diameter is about 1.1nm.
Uses of fullerene
Given its properties, Buckminsterfullerene finds a wide range of applications -
- It can be used in batteries, since it has the ability to both gain and lose electrons.
- Besides batteries, it also finds applications in some advanced electronic devices.
- Derivatives of C60 with alkali metals and alkaline earth metals find applications in superconducting materials.
- Can be used to reduce pollution caused by fossil fuels - finds application in the automotive industry.
- Can be used to enhance the properties of coating materials such as paints.
- Good lubricators because of their spherical molecular structure.
- Good oxidants. Derivatives of buckminsterfullerene are used in cosmetics.
Carbon Nanotubes
Unlike Buckminsterfullerene, Carbon nanotubes are cylindrical fullerene molecules. Their basic structure is a 6-Carbon ring.
 Fig. 3: Carbon Nanotube | Phys.org
Fig. 3: Carbon Nanotube | Phys.org
Like buckminsterfullerene, Carbon makes 3 covalent bonds in Nanotubes. This means there is a region of π electrons around their cylindrical shape. The diameter of Carbon nanotubes is usually only a few nanometres. However, they may vary from a few micrometres to a few millimetres in length. Nanotubes are the most recently discovered fullerenes and have since found many applications.
Carbon Nanotubes: Properties and Uses
Carbon nanotubes have the following properties:
- Excellent tensile strength.
- Excellent thermal conductivity - even better than diamond!
- Excellent electrical conductivity - multiple times better than even some metals like Copper.
These properties allow varied applications of Carbon nanotubes, such as -
- Used as reinforcing materials (for example in tennis rackets).
- Modification of composite materials. Can be used to make materials stronger and durable. Can also be used to make materials better thermal conductors.
- Can be used to make electrical components.
- Can be used in building materials.
- Can be used in the oil refining industry.
- Energy industry - Can be used in Lithium-ion batteries (batteries used in cell-phones and laptops!), solar cells, and fuel cells.
- Plastics - thermoplastics and thermosetting plastics.
- Can be used in nanotechnology.
- Their high tensile strength renders them as a potential use case in space elevators in the far future.
Fullerenes - Key takeaways
- Fullerenes are allotropes of Carbon.
- Allotropes are different structural forms the same element can exist in.
- Fullerene molecules have hollow structures.
- Fullerenes usually have hexagonal rings of Carbon for its basic structure, but can also have pentagonal rings of Carbon.
- Buckminsterfullerene (C60) is a fullerene with 60 Carbon atoms.
- Structure of Buckminsterfullerene is called truncated icosahedron. It is a spherical shape consisting of 20 hexagonal polygons, and 12 pentagonal polygons. Each vertex of each polygon has a Carbon atom; and each edge of each polygon is a bond between the Carbon atoms.
- Carbon nanotubes are fullerenes which have cylindrical shape.
- Carbon nanotubes have hexagonal rings of Carbon as their basic structure.
- In addition to the common properties of fullerenes, carbon nanotubes have high tensile strength, which makes them useful in many more applications.










