- The rate of the forward reaction and the rate of the backward reaction are equal.
- The concentrations of products and reactions remain the same.
But what if you want to know the composition of this equilibrium mixture? If you try to measure the amounts of products or reactants in the solution, it’s likely that you’ll end up disturbing the system. Instead, we can use the equilibrium constant. In this article, we’re going to focus specifically on the equilibrium constant Kc.
The equilibrium constant is a value that links the amounts of reactants and products in a reversible reaction at equilibrium.
- This article is about the equilibrium constant, Kc.
- We will begin by explaining what Kc is and go through the formula used to calculate it.
- We’ll then explore how we calculate the units for Kc and work through two examples together.
- Next, we’ll go through some exam questions so you can practice calculating Kc.
- After that, we'll look at what you can infer from the magnitude of Kc.
- Finally, we’ll touch on what factors affect the value of Kc.
What is the equilibrium constant Kc?
As we mentioned above, the equilibrium constant is a value that links the amounts of reactants and products in a mixture at equilibrium. There are a few different types of equilibrium constant, but today we’ll focus on Kc.
Kc is an equilibrium constant that links the concentration of reactants and the concentration of products in a reversible reaction at equilibrium.
There are two things to note when it comes to Kc:
- The higher the value of Kc, the higher the proportion of product compared to reactant at equilibrium.
- The value of Kc for a particular reaction at a certain temperature is always the same, no matter how much of the products or reactants you start with.
What is the equation for the equilibrium constant Kc?
Let’s take a general equilibrium reaction, shown below.
Kc measures concentration. This means that our products and reactants must be liquid, aqueous, or gaseous. To start with, we’ll look at homogeneous dynamic equilibria - these are systems in which all the reactants and products are in the same state. Later we’ll look at heterogeneous equilibria.
In this reaction, reactants A and B react to form products C and D in the molar ratio a:b:c:d.
Of course, because this is a reversible reaction, you could look at it from the other way - C and D react to form A and B. However, we’ll only look at it from one direction to avoid complicating things further.
What is the equation for Kc? Well, it looks like this:
Let’s break that down.
First of all, square brackets show concentration. So [A] simply means the concentration of A at equilibrium, in . And the little superscript letter to the right of [A]? That comes from the molar ratio. It means that we take the concentration of A and raise it to the power of the number of moles of A, that is given in the reaction equation.
Note that in the equation, the concentrations of the products are on the top of the fraction, and the concentrations of the reactants are on the bottom. We can also simplify the equation by removing the small subscript eqm from each concentration - it doesn’t matter, as long as you remember that you need concentration at equilibrium.
 Kc. Anna Brewer, StudySmarter Originals
Kc. Anna Brewer, StudySmarter Originals
Here’s an example.
What would the equilibrium constant for this reaction be?
- Take the equilibrium concentrations of the products.
- Raise them to the power of the molar ratio given in the equation.
- Divide them by the equilibrium concentrations of the reactants, raised to the power of the molar ratio given in the equation.
In this case, our only product is SO3. We have 2 moles of it in the equation. Our reactants are SO2 and O2. We have two moles of the former and one mole of the latter. Our equation for Kc should therefore look like this:
In this example, the reaction is an example of a homogeneous equilibrium - all the species are in the same state. How do we calculate Kc for heterogeneous equilibria?
Well, Kc involves concentration. You can’t really measure the concentration of a solid. If we have an equilibrium involving gases and a solid, for example, we just ignore the solid in the equation for Kc.
 Kc for a heterogenous reaction. Anna Brewer, StudySmarter Originals
Kc for a heterogenous reaction. Anna Brewer, StudySmarter Originals
Equilibrium constant units for Kc
The units for Kc can vary from calculation to calculation. It all depends on the reaction you are working with. You’ll need to know how to calculate these units, one step at a time.
- Take the equation for Kc.
- Replace the concentration of each species with the units that the concentration is measured in.
- Cancel units from the top and bottom of the equation until you are left with just one term.
Take our earlier example. All concentrations are measured in mol dm-3, so the equation now looks like this:
If we cancel them down, we end up with this:
Sometimes Kc doesn’t have any units. Look at this equation for a reversible esterification reaction:
If we find an equation for Kc, we get the following:
When we put the units in, we get (mol dm-3)(mol dm-3) on the top, and (mol dm-3)(mol dm-3) on the bottom. This cancels out to give 1, so there are no units:
Calculating the equilibrium constant Kc
In exam questions, you are usually given the initial concentrations of reactants. You are told about some aspect of the equilibrium solution and have to work out the concentrations of all the reactants and products at equilibrium. You can then work out Kc.
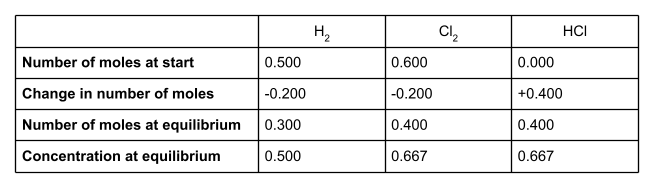
In a sealed container with a volume of 600 cm3, 0.500 mol H2 and 0.600 mol Cl2 react to form an equilibrium with the following equation:
At equilibrium, there is 0.400 mol HCl present in the container. Find a value for Kc. Include units in your answer.
First of all, let’s make a table. We’re going to use the information we have been given in the question to fill in this table. For each species, we’ll put the number of moles at the start of the reaction, the change in the number of moles, and the number of moles at equilibrium. You will also want a row for concentration at equilibrium.
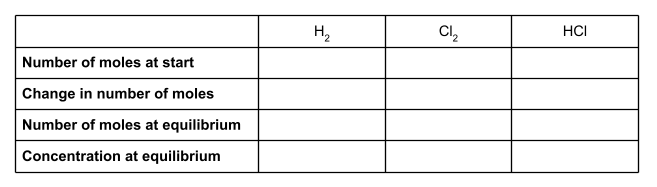
To start, write down the number of moles of all of the species involved at the start of the reaction. We were given these in the question. The question didn’t mention any moles of hydrochloric acid, so we can assume there wasn’t any.
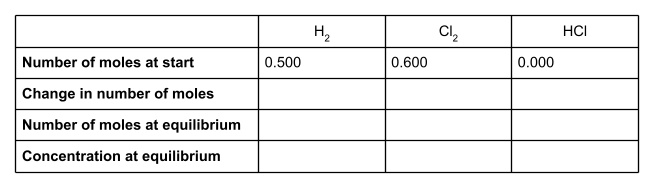
The question tells us that at equilibrium, there are 0.4 moles of HCl present. Write this value into the table.
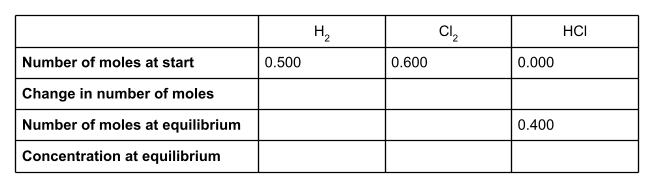
We can now work out the change in moles of HCl. At the start of the reaction, there wasn’t any HCl at all. At equilibrium, there are 0.400 moles. This is a change of +0.400.
If we take a look at the equation for the equilibrium reaction, we can see that for every two moles of HCl formed, one mole of H2 and one mole of Cl2 is used up. The molar ratio is therefore 1:1:2. To find out the number of moles of H2 and Cl2 used up in the reaction, divide the number of moles of HCl formed - the change in moles - by 2.
So 0.200 moles of H2 and 0.200 moles of Cl2 are used up in the reaction, to form 0.400 moles of HCl. The change in moles for these two species is therefore -0.200.
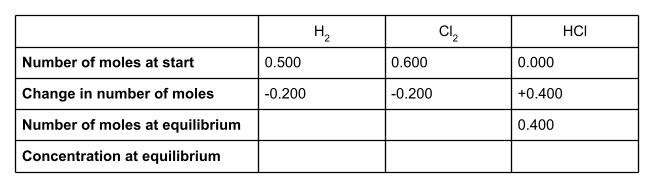
Once we know the change in number of moles of each species, we can work out the number of moles at equilibrium. To do this, add the change in moles to the number of moles at the start of the reaction.
Write these into your table.
Remember that Kc uses equilibrium concentration, not number of moles. But because we know the volume of the container, we can easily work this out.
Remember to turn your volume into . In this case, .
Concentration = number of moles volume
Your table should now be looking like this:
Now we can look at Kc. Remember that for the reaction . For our equation , Kc looks like this:
Notice that in the equation, the molar ratio of H2:Cl2:HCl is 1:1:2. In Kc, we must therefore raise the concentration of HCl to the power of 2.
Next, we can put our values for concentration at equilibrium into the equation for Kc:
The question gives all values to 3 significant figures, and so we must too.
The final step is to find the units of Kc. To do this, put the units of each of the concentrations into the equation for Kc and cancel them down. In this case, they cancel completely to give 1. Here, Kc has no units:
So our final answer is 1.33.
Here’s another question.
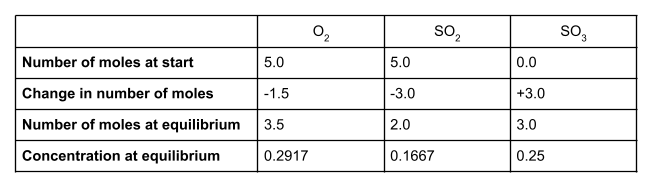
5.0 moles of O2 and 5.0 moles of SO2 reach dynamic equilibrium in a container of volume 12 dm3. The equilibrium contains 3.0 moles of SO3. Find Kc and give its units. The following equation may help you:
Let’s write out our table, as before:
At equilibrium, we have 3 moles of SO3. The change of moles is therefore +3. Because our molar ratio is 1:2:2, the change in moles for O2 must be -0.15 and the change in moles for SO2 must be -0.3. We can now work out the number of moles of each species at equilibrium and their concentrations, using the volume given of 12 dm3:
Your table should look like this:
The equation for Kc is as follows:
Subbing in our concentrations gives:
To find the units, we need to cancel the units of the concentrations down:
Our overall answer is therefore 7.7 mol-1 dm3.
Struggling to get to grips with calculating Kc? Here’s a handy flowchart that should simplify the process for you.
 A flowchart you can use to calculate Kc. Anna Brewer, StudySmarter Originals
A flowchart you can use to calculate Kc. Anna Brewer, StudySmarter Originals
Working backwards from Kc
Sometimes, you may be given Kc for a reaction and have to work out the number of moles of each species at equilibrium. This is a little trickier and involves solving a quadratic equation. Let’s work through an example together.
1 mole of ethyl ethanoate and 5 moles of water react together to form a dynamic equilibrium in a container with a volume of . The Kc for this reaction is 10.0. Find the number of moles of each substance at equilibrium, using the following equation to help you:
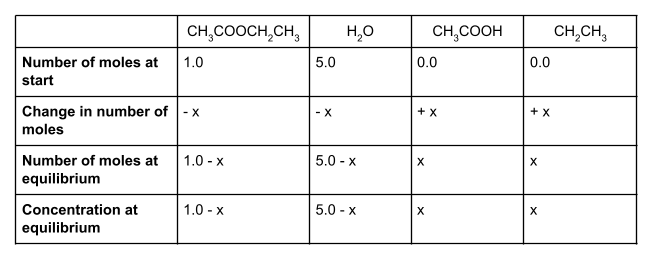
Let’s start by writing out the values that we do know in a table. We know that at the start, we have 1 mole of ethyl ethanoate and 5 moles of water. To form an equilibrium, some of the ethyl ethanoate and water will react to form ethanol and ethanoic acid.
We also know that the molar ratio is 1:1:1:1. For each mole of ethyl ethanoate that is used up, one mole of water will also be used up, forming one mole each of ethanol and ethanoic acid. However, we don’t know how much of the ethyl ethanoate and water will react. We can show this unknown value using the symbol x.
We started with 1 mole of ethyl ethanoate. If x moles of this react, then our equilibrium mixture will contain 1 - x moles of ethyl ethanoate. Likewise, we started with 5 moles of water. Because the molar ratio is 1:1:1:1, x moles of water will also react, and so the number of moles of water at equilibrium is 5 - x.
How much ethanol and ethanoic acid do we have at equilibrium? We started with 0 moles of each, and know from the molar ratio that we will produce x moles of each. This means that at equilibrium, we have exactly x moles of ethanol and x moles of ethanoic acid.
If you make a table showing all the values, it should look something like this:
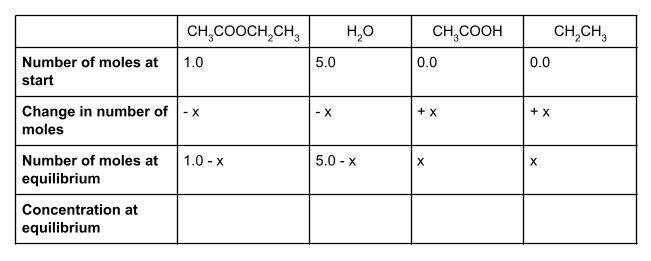
To find the concentration of each species at equilibrium, we divide the number of moles of each species at equilibrium by the volume of the container. In this case, the volume is 1 dm3. Anything divided by 1 gives itself, so here the equilibrium concentration is the same as the equilibrium number of moles.
Now let’s write an equation for Kc.
We can sub in our values for concentration. In the question, we were also given a value for Kc, which we can sub in too. This means that the only unknown is x:
Multiply both sides of the equation by (1-x) (5-x):
Expand the brackets to make a quadratic equation in terms of x and rearrange to make it equal 0:
You can now solve this using your calculator. You should get two values for x: 5.69 and 0.976. How do you know which one is correct?
Well, remember that x equals the number of moles of ethyl ethanoate and water that reacted to form a dynamic equilibrium. We only started with 1 mole of ethyl ethanoate. If 5.69 moles of ethyl ethanoate reacted, then we would be left with -4.69 moles, which isn’t possible - you can’t have a negative number of moles! Therefore, x must equal 0.976.
To finish this question, we can now find the number of moles of each species at equilibrium:
You might have noticed that we have only calculated Kc for homogeneous systems. These are systems where all the products and reactants are in the same state - for example, all liquids or all gases. However, we can calculate Kc for heterogeneous mixtures too if some of the species are solids. In these cases, the equation for Kc simply ignores the solids.
Take the following example:
For this reaction, . We ignore the concentrations of copper and silver because they are solids.
Magnitude of the equilibrium constant Kc
From the magnitude of Kc, we can infer some important things about the reaction at that specific temperature:
- If Kc is less than 1, then the denominator of the equation for Kc must be larger than the numerator. Therefore, we have a higher concentration of reactants than products at equilibrium. This means that the position of the equilibrium lies to the left and the backward reaction predominates.
- If Kc equals 1, then the numerator and the denominator of the equation for Kc must be the same. Therefore, we have equal concentrations of reactants and products at equilibrium. This means that the position of the equilibrium lies in the middle.
- If Kc is greater than 1, then the numerator of the equation for Kc must be larger than the denominator. Therefore, we have a higher concentration of products than reactants at equilibrium. This means that the position of the equilibrium lies to the right and the forward reaction predominates.
Factors affecting the equilibrium constant Kc
Finally, let’s take a look at factors that affect Kc. It’s actually quite easy to remember - only temperature affects Kc. Pressure, concentration and the presence of a catalyst have no effect on Kc whatsoever.
Take this example reaction:
If we decrease the temperature, the exothermic forward reaction will be favoured and thus the equilibrium will shift to the right. More of the product is produced, meaning its concentration increases, and thus the value of Kc also increases. Increasing the temperature favours the backward reaction and decreases the value of Kc.
Applications of the equilibrium constant Kc
The equilibrium constant Kc is useful because it allows us to manipulate the conditions of an equilibrium in order to influence the yield. One example is the Haber process, used to make ammonia. In fact, this is the reaction that we explored just above:
We know that at a certain temperature, Kc is always constant - its name is a bit of a giveaway. That means that at equilibrium, there will always be the same ratio of products to reactants in the mixture. In this case, our product is ammonia and our reactants are nitrogen and hydrogen.
Let’s say that we want to maximise our yield of ammonia. To do this, we can add lots of nitrogen and hydrogen gases to the mixture. This increases their concentrations. However, Kc says that the ratio of nitrogen and hydrogen to ammonia can’t change, so some nitrogen and hydrogen will be turned into ammonia to take the concentrations back to their equilibrium levels. The forward reaction is favoured and our yield of ammonia increases. This is just one example of an application of Kc.
Equilibrium Constants - Key takeaways
- Kc is a type of equilibrium constant that links the concentration of reactant and the concentration of product in a mixture at equilibrium.
- The higher the value of Kc, the higher the proportion of product in ratio to reactant at equilibrium.
- For the general reaction aA + bB ⇌ cC + dD,.
To find the units of Kc, you substitute the units of concentration into the equation for Kc and cancel them down.
To calculate Kc, you need to work out the number of moles of each species at equilibrium and their concentration at equilibrium.
The magnitude of Kc tells us about the equilibrium's position.
The value for Kc is affected by temperature but unaffected by concentration, pressure, and the presence of a catalyst.
Equilibrium constants allow us to manipulate the conditions of an equilibrium in order to increase its yield.
Similar topics in Chemistry
Related topics to Physical Chemistry



















