 Millions of hydrogen atoms can fit on the tip of a needle, so you can imagine how small they are! Pixabay.
Millions of hydrogen atoms can fit on the tip of a needle, so you can imagine how small they are! Pixabay.
Scientists can't spend all day staring at a microscope counting atoms, so we count them in groups based on Avogadro's number. We also use the unit of moles.
In this article, we will look at the many ways that Avogadro's number and the mole are important.
- This article is about Avogadro's number and the mole.
- We will look at an Avogadro's number example to calculate the number of atoms
- We will cover the importance of the mole, stoichiometry
- Lastly, we will learn to convert from grams to mols and from mols to atoms.
Avogadro's Number and the Mole Definition
Let's start by looking at the definition of Avogadro's number and the mole.
Avogadro's number was calculated by Jean Perrin in 1909, and was named after Amedeo Avogadro, who originally proposed the concept. It is equal to 6.022×1023, and is used in chemistry to count tiny objects like atoms or protons.
Avogadro's number is unitless. We can technically use it to count anything, but it is such a massive number that it is really only practical for small things like atoms or molecules. It is similar to how "a dozen" can refer to 12 things, but we typically use it just to count eggs. When we count atoms/molecules, we use the unit mole (not the animal, though they are cute!).
A mole (mol) is the standard unit used to count large amounts of atoms, molecules, or particles. 1 mol is equal to Avogadro's number.
Avogadro's Number and the Mole Importance
The mole is the standard way to count things in chemistry. It is used in our chemical formulas and equations, and it is used to convert from AMU (atomic mass unit) to the much more practical unit of grams.
The Mole, Avogadro's Number, and Stoichiometry
When we write chemical formulas and equations, the units we are using are mols. Let's look at a reaction.
$$2H_{2\,(g)} + O_{2\,(g)} \rightarrow 2H_2O$$
The coefficient (number in front) for each molecule is equal to the number of mols. If there is no coefficient written, it is 1. So in this case, we have 2 mols of H₂ and H2O and 1 mol of O2. The subscript (little number) tells us the number of moles per element. So for O2, there are 2 mols of atomic oxygen, O, per 1 mol of molecular oxygen, O2. Now that we know the basics, let's work on a problem.
$$Ba(OH)_{2\,(aq)}+2HCl_{aq} \rightarrow BaCl_{2\,(aq)}+2H_2O_{(l)}$$
1) How many O atoms are in Ba(OH)2?
When we have a parenthesis, we multiply the coefficient by the whole thing, so we have 2 mols of O atoms. Next, we know that 1 mol = 6.022 x 10 23, so we have 12.044 x 1023 atoms of O.
2) How many H atoms are in 2H2O ?
2) When counting total mols, we always need to multiply the subscript by the coefficient. There are 2 mols of H atoms per molecule, but since we have 2 mols of H2O and not 1, we actually have 4 mols of H. That means in total we have 2.4088 x 1024 (24.088 x 1023) atoms of H.
Chemical reactions must follow the law of conservation of mass. This law says that mass cannot be created or destroyed in a reaction (i.e. our products need to have the same mass as our reactants.) When writing chemical reactions, we have to abide by this law. To achieve this, we balance the number of moles for each element using stoichiometry (stoichiometry is just a fancy word for the ratio between products and reactants). Here's an example:
Balance the reaction:
$$C_3H_{8\,(g)} + O_{2\,(g)} \rightarrow CO_{2\,(g)} + H_2O_{(l)}$$
Let's start by counting the mols on each side.
On the left side (reactants) we have: 3 mols of C, 8 mols of H, and 2 mols of O
And on the right side (products): 1 mol of C, 3 mols of O (2 from CO2 and 1 from H2O), and 2 mols of H
So off the bat, we see that nothing is balanced. We can start by multiplying CO2 by 3 to balance our C
$$C_3H_{8\,(g)} + O_{2\,(g)} \rightarrow 3CO_{2\,(g)} + H_2O_{(l)}$$
Now we can balance H by multiplying H2O by 4
$$C_3H_{8\,(g)} + O_{2\,(g)} \rightarrow 3CO_{2\,(g)} + 4H_2O_{(l)}$$
Last is O. Now we have 10 O on our right (6 from CO2 and 4 from H2O), so we multiply O2 by 5 to get our final, balanced reaction
$$C_3H_{8\,(g)} + 5O_{2\,(g)} \rightarrow 3CO_{2\,(g)} + 4H_2O_{(l)}$$
When we do experiments, we are typically measuring in grams, not mols. So now we need to look at how we convert between these units.
The Atomic and Molar Mass
The way we convert from mols to grams (or vice-versa) is by using an element's atomic mass.
The atomic mass of an element is the average mass of all isotopes of an element measured in amu. 1 amu = 1 g/mol.
When we are referring to the mass of a whole compound, it is called the molecular weight/molar mass. The molecular weight is the sum of the atomic masses of each element in a compound. Being able to convert from moles to grams is important when experimenting for many reasons.
One reason is that we can calculate the yield of a reaction (mass of products), based on the mass of reactants. It is also important since we measure things in grams, but all of our chemical equations/formulas are in mols.
Given the compounds below, what is their molar mass?
a. SF6
b. Ba(OH)2
a.$$S:32.06 \frac{g}{mol}\,\,\,F:19.00 \frac{g}{mol}$$$$32.06 \frac{g}{mol}+(19.00 \frac{g}{mol}*6)=146.06 \frac{g}{mol}$$
b. $$Ba:137.33 \frac{g}{mol}\,\,\,O:16.00 \frac{g}{mol}\,\,\,H:1.01 \frac{g}{mol}$$
$$137.33 \frac{g}{mol}+(16.00 \frac{g}{mol}*2)+(1.01 \frac{g}{mol}*2)=171.35 \frac{g}{mol}$$
Using Avogadro's Number and the Mole Concept for Conversions
Now that we know how to calculate molecular weight, let's move on to converting from gram to mols and vice versaHow many mols of Fe3(PO4)2 is 143.2 g?
$$Fe: 55.85 \frac{g}{mol}\,\,\,P: 30.97\frac{g}{mol} \,\,\, O: 16.00 \frac{g}{mol}$$
$$(55.85\frac{g}{mol}*3)+(30.97\frac{g}{mol}*2)+(16.00\frac{g}{mol}*8)=357.49\frac{g}{mol}$$
$$\frac{143.2\,g}{357.49\frac{g}{mol}}=0.401\,mol$$
It's helpful to consider these conversions to be a ladder or staircase. You can either climb up or down, but you need to take each step to reach your destination.
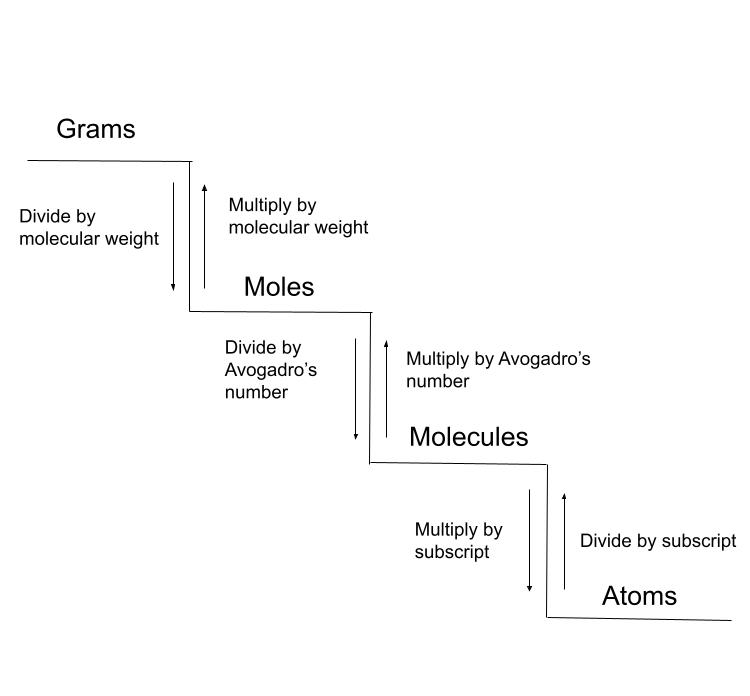
By following the "steps" we can convert between units. StudySmarter Original.
For some elements/compounds, the number of atoms is equal to the number of molecules. For example, in NaCl, both Na and Cl have no subscripts (so a subscript of 1), so these values are equal.
Percent Composition
Mols and molar mass can also be used to calculate percent composition.
Percent composition is the percentage of a compound that an element makes up by mass. The formula is:
$$\% \text{mass}=\frac{\text{mass of element in 1 mol}}{\text{molar mass of compound}}*100\%$$
Here's an example:
What is the percent composition of H and O in H2O?
The first thing we need to do is figure out the molar mass.
$$H: 1.01\frac{g}{mol}\,\,\,O: 16.00\frac{g}{mol}$$
$$(1.01\frac{g}{mol}*2)+16.00\frac{g}{mol}=18.02\frac{g}{mol}$$
Now that we have the total mass, we can determine each element's percent mass.
$$H: \frac{2.02\frac{g}{mol}}{18.02\frac{g}{mol}}*100\%=11.2\%\,\text{(because we have 2 H, we need to double the atomic mass)}$$
$$O: \frac{16.00\frac{g}{mol}}{18.02\frac{g}{mol}}*100\%=88.8\%$$
We can also use percent composition to calculate the mass of an element. So for our example above, H2O has an 11.2% composition of H:
$$\frac{11.2}{100}*18.02\frac{g}{mol}=2.02\frac{g}{mol}$$
When calculating percent composition, you should make sure your percentages add to 100. For the previous example, If we forgot to multiply the mass of H by 2, the total percentage would not have equaled 100.
Avogadro's Number and the Mole - Key takeaways
- Avogadro's number is equal to 6.022 × 1023 and is used to count things like molecules or atoms.
- 1 mole(mol) is equal to Avogadro's number and is the standard unit used to count molecules or atoms.
- The mol is important for writing and balancing chemical equations.
- We can use Avogadro's number and the mol to calculate the number of atoms or molecules when given grams.
- The formula for percent composition is: \(\% \text{mass}=\frac{\text{mass of element in 1 mol}}{\text{molar mass of compound}}*100\%\) It is used to determine what percentage an element makes up of a compound based on mass.









