So, what happened? What you witnessed is called a phase change. In this article, we will be learning all about phase changes and see why and how they occur.
This article is about phase changes.
- First, we will learn about the basics of phases and learn the definition of phase changes.
- Then, we will look at some examples of phase changes.
- Next, we will look at phase diagrams and learn how to interpret them.
- Lastly, we will learn about how energy is transferred during different phase changes.
Phase Change Definition
Matter can exist in several forms called phases or states. These are:
Solids
Liquids
Gases
The amount of energy within a species determines its phase. Below is a diagram showing what each phase looks like:
 Fig. 1 - Particles are spaced differently in each state of matter
Fig. 1 - Particles are spaced differently in each state of matter
Here's a breakdown of what each illustration means:
- Solid particles are held tightly in fixed positions and are in constant contact with one another. They do have enough energy to vibrate in place.
- Liquid particles are still close together and in constant contact, but have enough energy to switch positions with each other.
- Gas particles have enough energy to move freely. They only come in contact occasionally when they collide with each other.
When species either gain or lose enough energy, they can go through a phase change.
A phase change is a physical process where a substance changes state. This change usually occurs at a temperature called the boiling point or the melting point.
The boiling point is the temperature where a liquid becomes a gas OR a gas becomes a liquid (instead called condensation point). If a liquid gains enough energy for the molecules to separate, it is boiling. If a gas loses energy to reform into a more confined state, it is condensing.
The melting point is the temperature where a solid becomes a liquid OR a liquid becomes a solid (instead called freezing point). If a solid gains enough energy to disrupt the forces between particles, it is melting. If a liquid loses enough energy for these forces to strengthen enough to reform, it is freezing.
Since these points are where a phase change occurs, both phases (solid/liquid or liquid/gas) can exist at that temperature. We will talk about this in more detail later.
There is a fourth, less common state of matter called plasma. Plasma has the highest energy of all the states of matter. In the plasma form, electrons wander around the different nuclei of the atoms. The formation of plasma is often caused by the ionization of a gas. The transition of plasma to a gas is called recombination.
Sublimation Phase Change
While most phase changes involve a liquid at some point, there are a couple of phase changes that skip the liquid phase entirely. One of them is known as sublimation.
Sublimation is the process of a solid becoming a gas without first changing into a liquid. The reverse process (gas to solid) is called deposition.
You've probably seen sublimation in action if you have ever seen "dry ice" (solid CO2). At room temperature, dry ice sublimes from a solid to a gas, which is why it appears as smoke.
Phase Change Examples
Now that we've learned the basics of phase changes, let's look at some examples. There are 6 phase changes in total:
Melting (solid \( \rightarrow \) liquid)
Solidifying/Fusion (liquid \( \rightarrow \) solid)
Boiling (liquid \( \rightarrow \) gas)
Condensing (gas \( \rightarrow \) liquid)
Sublimation (solid \( \rightarrow \) gas)
Deposition (gas \( \rightarrow \) solid)
Phase Change Diagram
Interestingly, when a phase change occurs, the temperature doesn't change. Instead, whatever heat is being added\subtracted is going into making the phase change happen.
Below is the phase change diagram for water:
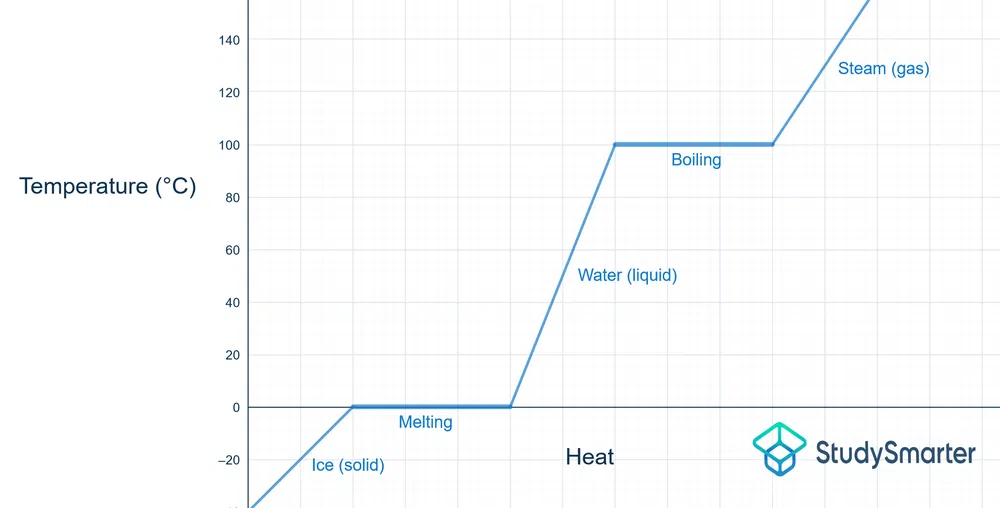
Fig. 2 - Diagram showing the change in phase and temperature for water
The melting point of water is 0 °C, and you can see that once that temperature is reached, it flat-lines for a bit. The heat that is being added is used to move the ice particles apart until they become water (i.e., liquid phase).
The same process occurs at 100 °C, the boiling point of water. The heat energy being added is pulling apart the water molecules, so they can become steam (i.e., gaseous phase).
When the reverse is occurring (liquid to solid, gas to liquid), the temperature is still unchanged as the phase change h. Heat/energy is being released, so the particles end up closer together.
As mentioned previously, at the boiling point/melting point, both phases can exist at that temperature. Once you hit the needed temperature, a certain amount of heat needs to be added for a complete phase change. Before that happens, there exists either a solid+liquid or a liquid+gas, as one is being converted into the other.
The temperature at which something boils/melts is dependent on pressure. When we refer to the boiling/melting point of water, we are referring to these points at 1 atm (atmosphere at sea level). However, the boiling/melting point will change depending on the pressure.
Below is a phase diagram for CO2 (carbon dioxide).
 Fig. 3 - Phase changes for carbon dioxide.
Fig. 3 - Phase changes for carbon dioxide.
The border between each phase represents where that phase change will occur. At standard pressure (1 atm), and room temperature (~20 °C), carbon dioxide is a gas/vapor, but at very low temperatures it is solid. This explains why dry ice sublimes instead of melts The graph above also shows us two important points:1. The triple point2. The critical point
The triple point is where the three phases coexist in equilibrium.
Essentially, the triple point is a crossroads, where you can choose any direction (to get to any phase). Let's say we had a sample of dry ice at 5.2 atm and -100 °C. As the temperature increases and reaches -57 °C, the dry ice will start to convert into both liquid and gaseous CO2.
If the temperature continues to increase, the dry ice will be completely converted into a gas. However, if the pressure increases, the dry ice would instead become a liquid.
The critical point is where a species becomes a supercritical fluid.
A supercritical fluid is a substance where there is no distinction between the liquid and gas phases.
The critical point is essentially the "endpoint" for the boiling point curve (border between liquid and gas). After this point, boiling cannot occur. The species has combined characteristics of a liquid and gas, so it is difficult to differentiate between the two. Converting from a liquid to a gas cannot happen, since the species isn't a liquid or a gas anymore.
Energy of Phase Changes
Each phase change has its own energy associated with it. For all molecules/elements, it takes a set amount of energy for a phase change to occur. The heat required for boiling to occur is called the heat of vaporization (ΔHvap), while the heat required for melting is the heat of fusion (ΔHfus).
While the magnitude is the same, the signs of each are dependent on the direction of the phase change. If heat is being added, ΔHvap and ΔHfus are positive, while they are negative if heat is being released.
These values are dependent on the interactions between molecules. If molecules have strong forces between them, it will take more energy to change their state.
Here is a table of some common heats of vaporization/fusion values.
| Name of substance | ΔHfus (J/g) | ΔHvap (J/g) |
| Water | 334 | 2260 |
| Ethanol (C2H5OH) | 109 | 838 |
| Oxygen | 14 | 213 |
| Methane (CH4) | 59 | 537 |
| Iron | 209 | 6340 |
| Nitrogen | 25.5 | 200 |
The total heat required is based on the mass of the species. The formula for the heat change is:
$$ \text{heat}=m*\Delta H_{x} \quad or \quad \text{heat}=n*\Delta H_{x} $$
Where m is the mass of the species, n is the number of moles of the species and \(\Delta H_{x}\) is either the heat of vaporization or the heat of fusion
Let's try an example problem:
It takes 679 J to vaporize a sample of liquid nitrogen. If the ΔHvap = 200 J/g, how many grams of nitrogen were in the sample?
The formula we would use is: $$\text{heat}=m*\Delta H_{vap}$$ To get the mass of the sample, we need to divide the heat required to vaporize the sample by the heat of vaporization.
$$\frac{\text{heat}}{\Delta H_{vap}}=m$$
$$\frac{679\,J}{200\frac{J}{g}}=m$$
$$m=3.4\,g$$
Let's do one more example using the other formula:
It takes 11,239 J to melt a sample of gallium. If the ΔHfus = 5.59 kJ/mol, how many moles of gallium were in the sample?
Since we are looking for the number of moles in a melted sample, we would use this formula:
$$\text{heat}=n*\Delta H_{fus}$$
Firstly, we need to convert our heat into kJ, since that is the unit our heat of fusion is in.
$$11,239\,J*\frac{1\,kJ}{1000\,J}=11.239\,kJ$$
Now we can plug in our variables and solve for n (number of moles)
$$\text{heat}=n*\Delta H_{fus}$$
$$n=\frac{\text{heat}}{\Delta H_{fus}$$
$$n=\frac{11.239\,kJ}{5.59\frac{kJ}{mol}}$$
$$n=2.01\,mol$$
Endothermic Phase Changes
As we mentioned earlier, phase changes are caused by either a gain or release of heat. Phase changes caused by a gain of heat are called endothermic phase changes (ΔH > 0). These are:
Boiling (liquid --> gas)
Melting (solid --> liquid)
Sublimation (solid --> liquid)
Exothermic phase changes are the opposite. Heat is released during these phase changes (ΔH < 0). These are:
Condensing (gas --> liquid)
Solidifying (liquid --> gas)
Deposition (gas --> solid)
Phase Changes - Key takeaways










