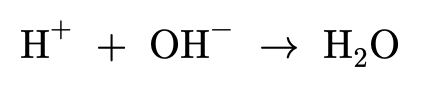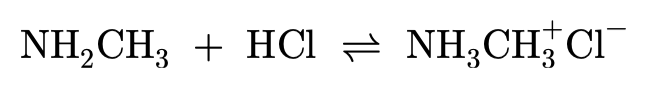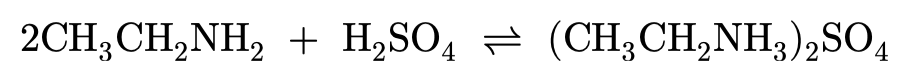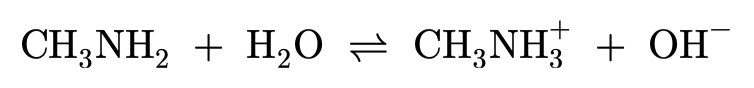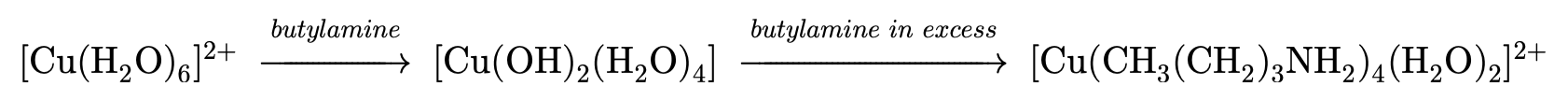
Nucleophilicity describes the ability of a chemical or compound to act as a nucleophile. The definition of a nucleophile is an electron-pair donor. So in a chemical reaction, the nucleophile would form covalent bonds by donating a pair of electrons to electron-poor sites.
In both cases, whether an amine is acting as a base or a nucleophile, there will be a bond formed between the lone nitrogen pair and something else. Just remember:
- if the amine is bonding to a hydrogen ion, then it's acting as a base
- if the amine is bonding to anything other than a hydrogen ion, then it's acting as a nucleophile.
Why do amines act as bases?
Amines contain a lone pair of electrons on the nitrogen and are therefore able to form dative covalent bonds (which you can learn more about in Covalent Bond). When amines bond with hydrogen ions they become 'proton acceptors', allowing amines to act as a weak base. For a molecule to act as a base it must be able to react with acids, which are proton donors.
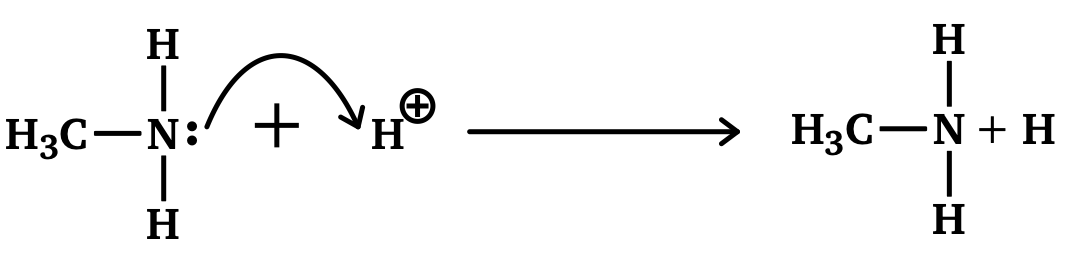
Amines and conjugate acids:
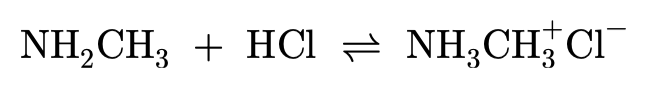
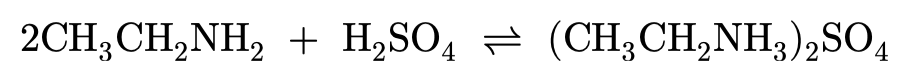
Small amines and water:
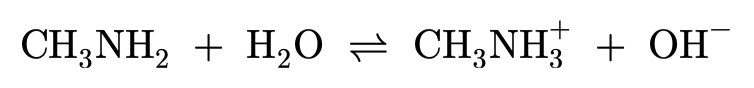
Amines and copper (II) ions: (Fehling's solution is a blue solution of copper (II) ions dissolved in sodium hydroxide)
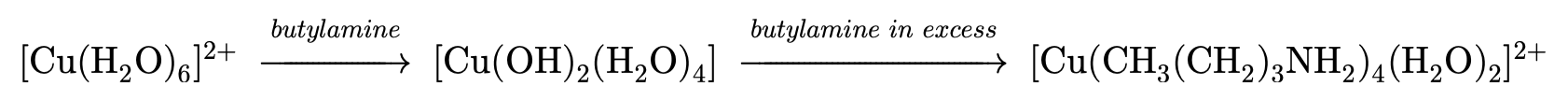
How strong are amines as bases?
Amines act as weak bases, meaning they will only partially dissociate in water.
When an amine acts as a base, the strength of its basicity depends on the type of amine; aromatic or aliphatic. The lone pair on the nitrogen gives amines their basic properties by 'accepting' and bonding with protons, so the availability of nitrogen's lone pair determines the likelihood of accepting a proton and therefore the strength of the base.
- If the lone pair is more available, the amine is a stronger base.
- If the lone pair is less available, the amine is a weaker base.
Lone pairs become more available when the electron density of an amine is concentrated around the nitrogen atom. Let's see how the type of amine affects the availability of the lone pair.
Benzene ring
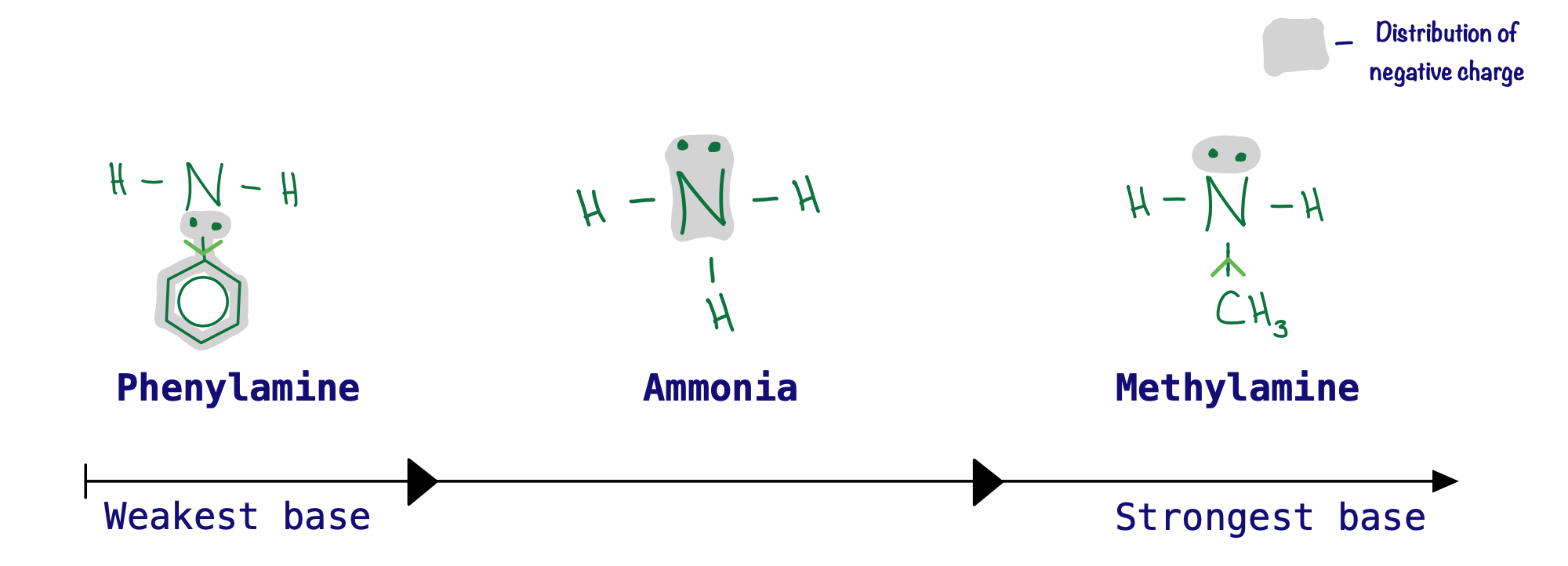
Aromatic amines have a benzene ring bonded to the nitrogen. Benzene rings draw electrons towards them, shifting the electron density away from the nitrogen, and the lone pair from the nitrogen actually becomes 'partially delocalised' onto the ring. The lone pair will therefore be much less available, meaning aromatic amines have the weakest basicity.
Alkyl groups
Aliphatic amines have at least one alkyl group bonded to the nitrogen atom. Alkyl groups 'push' electrons onto the nitrogen, having the opposite effect to benzene. By pushing electrons onto the nitrogen, there is a higher electron density around the nitrogen which means the lone pair will be more available. Therefore, aliphatic amines are stronger bases than aromatic amines and ammonia.
Ammonia
The nitrogen atom in ammonia experiences no inductive or delocalised effect on its electrons. For this reason, ammonia sits in between aromatic and aliphatic amines when it comes to its basic strength.
Primary, secondary, and tertiary amines
The difference in basic strength between primary, secondary, and tertiary amines is a bit more complicated. In general, primary amines are always weaker than secondary and tertiary amines, but the difference between secondary and tertiary amines is also dependent on the state of matter.
In their gaseous states, tertiary amines are more basic than secondary amines, but when they are in an aqueous solution it's reversed; secondary amines are stronger bases than tertiary amines. (Knowing the reasons for this isn't necessary at this level.)
Which reactions involve amines as the nucleophile?
The lone pair of electrons on nitrogen allow amines to act as nucleophiles.
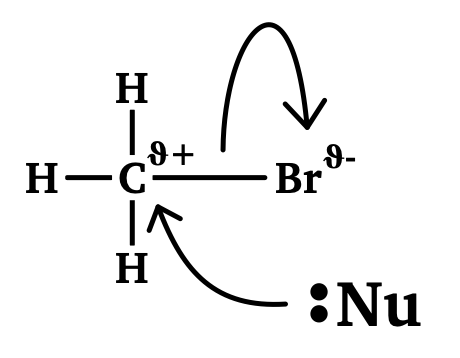
Nucleophilic substitution
Amines act as nucleophiles when they react with halogenoalkanes in a nucleophilic substitution reaction (See Halogenoalkanes and Nucleophilic Substitution Reactions for more information). In this reaction, the amine forms a covalent bond with a carbon atom and replaces the bonded halogen. The outcome of this reaction is a quaternary ammonium ion salt and an amine that has gained another organic group.
Nucleophilic addition-elimination
Amines react with acyl chlorides or acid anhydrides in a nucleophilic addition-elimination reaction.
- When amines react with acyl chlorides they form an N-substituted amide and hydrogen chloride gas.
- When amines react with acid anhydrides they form an N-substituted amide and a carboxylic acid.
Amines Basicity - Key takeaways
- Amines act as bases by forming dative covalent bonds with protons from the lone pair of electrons on the nitrogen atom.
- This can be demonstrated in the reactions between amines and conjugate acids, amines and water, or amines and copper (II) ions.
- The basic strength of an amine depends on the availability of nitrogen's lone pair.
- Aromatic amines are the least basic because the electron density in the benzene ring causes nitrogen's lone pair to become partially delocalised.
- Ammonia is more basic than aromatic amines because there is no partial delocalisation of nitrogen's lone pair.
- Aliphatic amines are more basic than aromatic amines and ammonia because the alkyl groups have an inductive effect on nitrogen's lone pair.
- Amines act as nucleophiles when the lone pair on the nitrogen atom attacks other organic molecules with areas of partial positive charge.
- Reactions in which an amine will act as a nucleophile include nucleophilic substitution reactions with halogenoalkanes, and nucleophilic addition-elimination reactions with acyl chlorides or acid anhydrides.